Carpet and Upholstery Cleaning Agents: Understanding Chemical Concentration
Cleaning Agents: Impact of Concentration on Carpet and Upholstery Cleaning Results.
Introduction
The science of carpet and upholstery cleaning may often be disregarded, but it is crucial for achieving exceptional cleaning outcomes for homeowners and professionals alike. By understanding cleaning agents it becomes possible to unveil the hidden secrets behind immaculate carpets and upholstery. Here we will focus on the interplay of chemical concentrations and the various reactions that occur during cleaning,
This authoritative, and engaging article delves into the vital impacts of concentration, temperature, time, and agitation on these chemical processes. It also demonstrates the importance of employing diverse cleaning agents for different soil types. By demystifying the science of carpet and upholstery cleaning, you will be well-prepared to maintain a spotless environment for your home or business. From the ancient era of carpet cleaning to the modern era, it has improvised a lot.
Chemical Concentration in Cleaning Agents
Balancing cleaning efficiency and safety
The right concentration of cleaning agents is crucial for efficient cleaning and maintaining the fabric’s integrity.
Over-concentration can damage the material, while under-concentration leads to poor cleaning results.
Types of cleaning agents and their functions
Detergents are surfactants and emulsifiers that help remove dirt and grime.
Solvents dissolve oil-based and water-based soils for easy removal.
Enzymes break down organic materials, such as proteins, fats, and carbohydrates, in stains and odors.
Acids and alkalis neutralize and dissolve mineral deposits and stains, helping in the cleaning process.


Understanding Chemical Concentration in Cleaning Agents for Optimal Results
Striking the Right Balance between Cleaning Efficiency and Safety
Achieving the correct concentration of cleaning agents is critical for effective cleaning while also ensuring the safety of your carpet or upholstery. Using too much of a cleaning agent can damage the material and leave residue, while using too little can lead to inadequate cleaning results. By finding the perfect balance, you can ensure a deep clean without compromising the fabric’s integrity.
Different Types of Cleaning Agents and Their Functions
Understanding the various types of cleaning agents and their functions is crucial for achieving a thorough and efficient clean. By choosing the appropriate cleaning agent for your specific carpet or upholstery cleaning needs, you can ensure your space looks and feels fresh. Let’s explore four common cleaning agents and their roles in the cleaning process.
To understand how these cleaning agents work, let’s delve deeper into the mechanisms behind each type of agent and their interactions with soils and stains.
Detergents: Powerful Surfactants and Emulsifiers
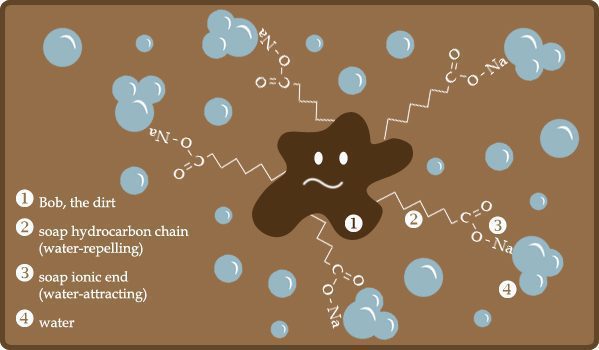
Detergents contain surfactants, which are molecules with a dual nature – one end is hydrophilic (water-attracting) and the other end is lipophilic (oil-attracting). When you apply a detergent to a stain, the lipophilic end of the surfactant molecule attaches itself to the dirt or grease particles, while the hydrophilic end remains in the water. This action effectively breaks up the soil particles and prevents them from reattaching to the carpet or upholstery fibers4. Emulsifiers in detergents help to keep the soil particles suspended in the cleaning solution, making it easier to rinse or extract them away.
You need to keep in mind about fiber distortion while using any kind of cleaning agents.
Solvents: Effective Dissolvers of Soils
Solvents work by a process called solvation, in which they interact with and dissolve various types of soils. Polar solvents are effective at dissolving polar soils, such as water-based stains, while non-polar solvents are more effective at dissolving non-polar soils, like oil-based stains3. Solvents weaken the chemical bonds holding the soil particles together, allowing the particles to disperse in the solvent and be removed more easily during the cleaning process.
Enzymes: Powerful Stain and Odor Breakdown Agents
Enzymes are proteins that act as biological catalysts, meaning they speed up the rate of chemical reactions. They work by breaking down complex organic materials found in household stains, such as proteins, fats, and carbohydrates, into simpler, more manageable components1. For example, protease enzymes break down proteins into smaller peptides and amino acids, while lipase enzymes break down fats into glycerol and fatty acids. By breaking down these complex materials, carpet cleaning enzymes make it easier to remove the stain and neutralize any associated odors.
Acids and Alkalis: Neutralizers and Dissolvers of Mineral Deposits and Stains
Acids and alkalis work by altering the pH of the cleaning solution, which can help to dissolve and neutralize mineral deposits and stains. Acidic cleaning solutions are effective at dissolving mineral deposits like hard water stains, calcium, and rust, while alkaline cleaning solutions are better suited for breaking down and neutralizing acidic stains, such as red wine or coffee3. By altering the pH of the cleaning solution, acids and alkalis can weaken the chemical bonds holding the stain particles together, allowing them to be more easily removed during the cleaning process.
In summary, detergents, solvents, enzymes, acids or alkalis each have unique mechanisms that enable them to break down and remove various types of soils and stains. By understanding how these cleaning agents work, you can choose the most effective agent for your specific carpet or upholstery cleaning needs, ensuring a thorough and efficient clean.


Chemical Reactions in the Cleaning Process
Unraveling the Chemical Reactions in the Carpet and Upholstery Cleaning Process
Surfactants and Emulsification
Surfactants are essential components of cleaning agents, working to reduce surface tension and improve wetting4. This allows the cleaning solution to better penetrate the fabric, reaching deep into the fibers to remove stubborn dirt and grime.
Emulsification is another crucial aspect of surfactants, which helps break down oily soils, making them easier to remove from carpets and upholstery. For example, surfactants can efficiently tackle grease stains in high-traffic areas of your home or office.
Solvent-based Cleaning
Solvent-based cleaning relies on the process of solvation, where solvents dissolve various soils, making them easier to extract from carpets and upholstery5.
Polar solvents are particularly effective at removing water-based stains, like juice spills, while non-polar solvents are better suited for tackling oil-based stains, such as those caused by cooking oil.
By understanding the differences between polar and non-polar solvents, you can choose the most effective cleaning agent for each type of stain.
Polar and non-polar solvents play a crucial role in removing different types of stains, such as water-based and oil-based stains.
Enzymatic Reactions
Enzymatic reactions play a critical role in breaking down organic materials like proteins, fats, and carbohydrates, thus facilitating the removal of organic stains and odors1.
These cleaning agents are particularly useful for tackling stubborn organic stains, such as those caused by food spills and pet accidents. For instance, enzyme-based cleaners can help eliminate lingering odors from pet urine or neutralize stubborn food stains on your favorite sofa.
Acid and Alkali Reactions
Acid and alkali reactions are responsible for neutralizing and dissolving mineral deposits and stains in carpets and upholstery3.
The pH level of cleaning agents plays a vital role in their effectiveness and safety, with acidic cleaners targeting mineral-based stains like rust and alkaline cleaners working on organic and protein-based stains. By carefully considering the pH level of your chosen cleaning agents, you can ensure their effectiveness while protecting your carpets and upholstery from potential damage.
The Effects of Concentration, Temperature, Time, and Agitation on Chemical Reactions
Concentration
Increasing the concentration of cleaning agents improves cleaning efficiency.
Over-concentration poses risks, such as damage to carpets and upholstery, residue buildup, and environmental concerns.
Temperature
Temperature speeds up chemical reactions, as explained by the Arrhenius equation. Optimal temperature ranges vary for different cleaning agents and methods. Precautions should be taken to avoid potential damage caused by excessive heat during the cleaning process.
Time
Dwell time is essential for chemical reactions to occur, ensuring effective cleaning. Balancing dwell time helps achieve efficient cleaning while minimizing potential damage to carpets and upholstery.
Agitation
Agitation accelerates chemical reactions, improving cleaning results1. Appropriate agitation methods should be selected based on carpet and upholstery materials and conditions to avoid damage.
Understanding the Impact of Concentration, Temperature, Time, and Agitation on Carpet and Upholstery Cleaning
Concentration: The Key to Efficiency and Safety
Increasing the concentration of cleaning agents in your carpet and upholstery cleaning solution can boost the effectiveness of the cleaning process, as a higher concentration helps break down and dissolve dirt and stains more efficiently.
However, using too much cleaning agent can lead to several problems, such as damaging your carpets and upholstery, leaving behind residue, and causing harm to the environment. To prevent these issues, always follow the manufacturer’s recommended concentrations for optimal cleaning results6.
The Role of Temperature in Chemical Reactions
Temperature plays a crucial role in the speed of chemical reactions during the carpet and upholstery cleaning process. The Arrhenius equation explains that higher temperatures typically lead to faster chemical reactions, resulting in more effective cleaning5.
Different cleaning agents and methods work best within specific temperature ranges. Ensuring that you use the optimal temperature for each cleaning solution will maximize efficiency and effectiveness3.
However, it’s essential to be cautious and avoid using excessive heat during the cleaning process, as this can damage carpets and upholstery. Always consult the manufacturer’s guidelines to determine the appropriate temperature settings for your cleaning equipment.
Time: Striking the Right Balance
Dwell time, or the amount of time that cleaning agents are in contact with carpets and upholstery, is crucial for chemical reactions to occur and ensure effective cleaning. Allowing enough time for cleaning solutions to work on dirt and stains is key to achieving a thorough clean.
However, it’s important to balance dwell time, as leaving cleaning solutions on carpets and upholstery for too long can result in potential damage, such as discoloration or weakened fibers. To prevent these issues, adhere to the recommended dwell times provided by cleaning solution manufacturers6.
Agitation: Accelerating Chemical Reactions and Improving Cleaning Results
Agitation, or the mechanical action used to distribute cleaning agents and help dislodge and suspend dirt and stains, can significantly accelerate chemical reactions and improve cleaning results. Effective agitation ensures that cleaning solutions penetrate deeply into carpet and upholstery fibers, promoting better overall cleaning3.
To avoid causing damage, it’s essential to choose the right agitation method for your carpet and upholstery materials and conditions. For instance, delicate fabrics may require gentle brushing or scrubbing, while heavily soiled areas may benefit from more aggressive agitation techniques, such as extraction.
Understanding the effects of concentration, temperature, time, and agitation on chemical reactions in carpet and upholstery cleaning can help you make informed decisions and achieve the best possible cleaning results. By following these guidelines and selecting the appropriate cleaning agents and methods, you can ensure a clean, fresh, and well-maintained living space.
Determining Chemical Concentration Using Empirical Formulas
The empirical formula of a cleaning agent represents the simplest ratio of elements in a compound. It provides crucial information regarding the proportion of different elements present in the cleaning solution. To determine the appropriate concentration for carpet and upholstery cleaning, you’ll need to consider the empirical formula of the cleaning reagents and adjust the concentration according to the manufacturer’s guidelines.
For instance, let’s say you have a cleaning agent with an empirical formula of C6H12O6 (glucose). This formula indicates that the compound contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. Based on this information, you can calculate the compound’s molecular weight and use this information to create a solution with the desired concentration.
However, it is essential to follow the manufacturer’s recommendations for the appropriate concentration, as they have conducted extensive research and testing to determine the optimal concentration for various cleaning situations. Over- or under-concentrating the cleaning solution can lead to reduced cleaning efficiency or potential damage to the carpets and upholstery.
Effective Time for Carpet and Upholstery Cleaning Reactions
The dwell time, or the time it takes for the cleaning solution to effectively break down and dissolve soils, depends on various factors, including the cleaning agent type and the soil’s nature. Generally, a dwell time of 10-20 minutes is considered effective for most carpet and upholstery cleaning applications3. However, always refer to the manufacturer’s guidelines for the specific cleaning solution you are using to ensure optimal results and prevent potential damage.
Appropriate Temperature Range for Cleaning Carpet and Upholstery
The optimal temperature range for carpet and upholstery cleaning varies depending on the cleaning agent and method used. Generally, a temperature range of 120-160°F (49-71°C) is considered appropriate for most hot water extraction methods5. Some cleaning solutions, especially enzyme-based cleaners, may require lower temperatures (around 60-100°F or 16-38°C) to maintain enzyme activity and prevent denaturation1.
It is crucial to consult the manufacturer’s guidelines for the specific cleaning solution and equipment you are using to determine the appropriate temperature settings for your carpet and upholstery cleaning needs.

Conclusion
Grasping the nuances of chemical concentration and reactions is essential for optimal carpet and upholstery cleaning.
Understand how factors like concentration, temperature, time, and agitation affect chemical reactions in cleaning. Choose the right cleaning agents for different soils and materials. This approach preserves the beauty and life of your carpets and upholstery.
While carpet and upholstery cleaning seems straightforward, its science is detailed. Understanding the principles in this article prepares you to effectively handle tough stains and dirt.
Well-kept, clean carpets or upholstery not only improve your space’s look but also promote a healthier living environment.
To conclude, a deep understanding of chemical concentration and reactions in cleaning is key to excellent results. Being conscious of how concentration, temperature, time, and agitation influence these reactions enables informed choices of cleaning agents and methods for diverse soils and materials. This insight helps maintain your carpets and upholstery’s condition, fostering a clean, inviting atmosphere.
References:
1 Bryant, R. (2019). Enzymes in Cleaning Products. Green Living Ideas. Retrieved from https://greenlivingideas.com/2019/01/09/enzymes-in-cleaning-products/
2 Church & Dwight Co., Inc. (2019). The Science of Clean: Understanding Cleaning Product Formulations. Arm & Hammer. Retrieved from https://www.armandhammer.com/articles/science-of-clean-understanding-cleaning-product-formulations
3 Institute of Inspection, Cleaning and Restoration Certification. (2012). IICRC S100 Standard and Reference Guide for Professional Carpet Cleaning (2nd Edition). Vancouver, WA: IICRC.
4 Rosen, M. J., & Kunjappu, J. T. (2012). Surfactants and Interfacial Phenomena (4th Edition). Hoboken, NJ: John Wiley & Sons
5 Ratcliff, C. (2005). Chemistry of Cleaning. Cleanfax. Retrieved from https://cleanfax.com/carpet-care/chemistry-of-cleaning/
6 Carpet and Rug Institute. (2011). CRI Carpet Cleaning Tips for Dummies. Retrieved from https://carpet-rug.org/carpet-cleaning-tips-for-dummies/
